In the case of tirzepatide, its mode of action as a dual agonist is based on the fact that it acts on two receptors in the body: the GLP-1 receptor (glucagon-like peptide-1 receptor) and the GIPR receptor (glucose-dependent insulinotropic polypeptide receptor). An international research team led by junior research group leader Johannes Broichhagen from Leibniz-FMP, David Hodson from the Radcliffe Department of Medicine at the University of Oxford and Anne de Bray from the University of Birmingham has now succeeded in using new fluorescent markers to visualize the journey of drugs such as tirzepatide through the body and brain. The results have now been published in the journal Nature Metabolism. “By describing the cell targets for dual agonists, we can better understand how they exert their effects”, says David Hodson from the Radcliffe Department of Medicine, University of Oxford UK. Based on these findings, it is possible to further improve the treatment of diabetes and obesity.
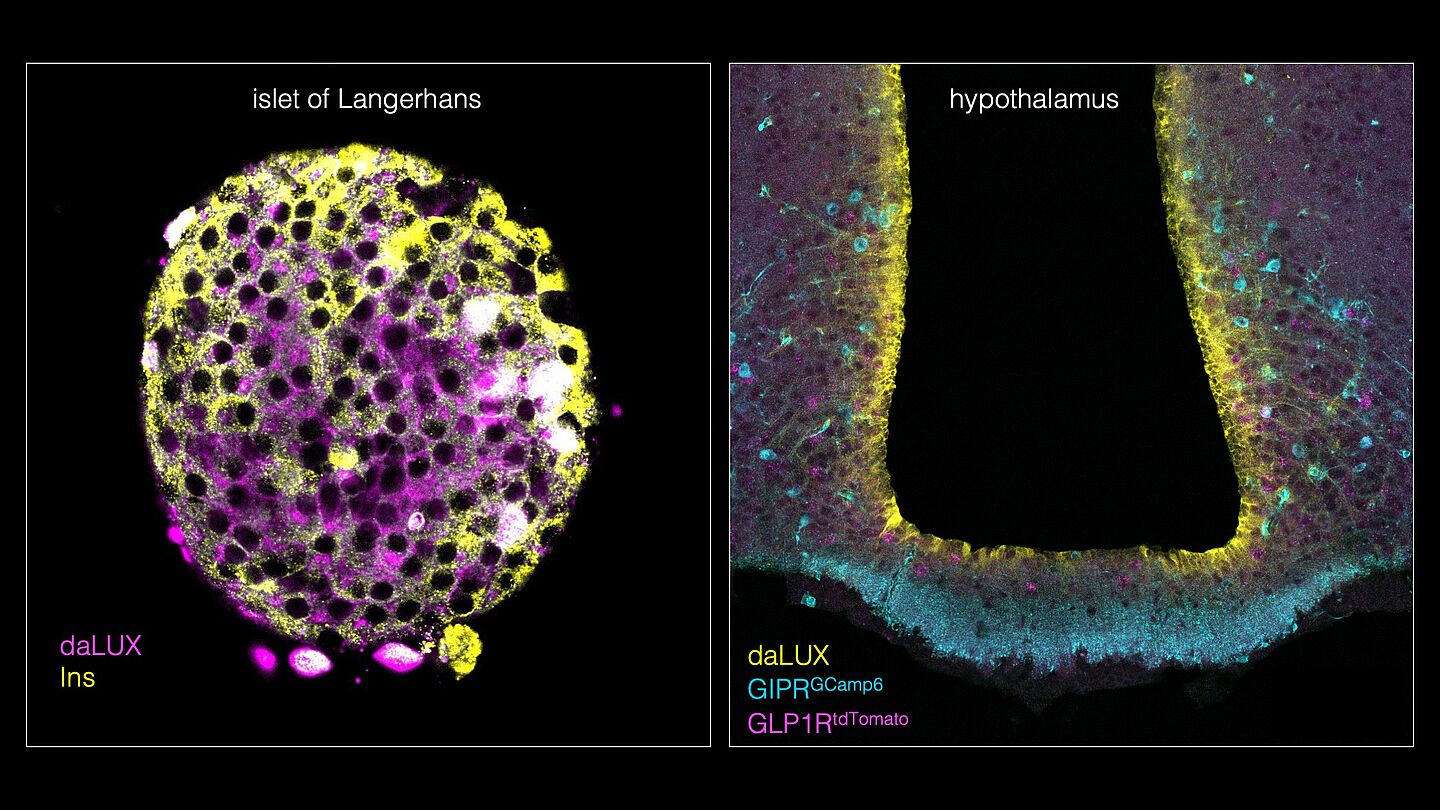
GLP1 and GIP receptors are found in both the pancreas and the brain. They ensure that enough insulin is released after a meal. While the mode of action of tirzepatide in the body has already been well researched, it was previously unclear exactly which specific cell and nerve types are targeted by the drug. The research team led by junior research group leader Johannes Broichhagen has now gained new, decisive insights in this area: after years of work, they have succeeded in developing novel fluorescent markers using fluorophores (dual agonist LUXendins or daLUXendins). These enable the visualization and analysis of both GLP1R and GIPR receptors simultaneously in living cells and tissues, thus revealing the target cells of dual agonists such as tirzepatide.
Conventional methods such as antibodies are not sufficient for detection because they are sometimes not validated or are not even available for specific proteins. Furthermore, they do not provide answers as to how and where drugs act on the pancreas and in the brain. ‘We are particularly interested in the dynamics of the peptides,’ says Johannes Broichhagen. Experiments with daLUXendins have shown that the markers bind most strongly to beta cells, but also to alpha and delta cells in the pancreas. It has also been demonstrated that tirzepatide reaches specific brain regions and nerve cells that are important for controlling appetite and metabolism. A particularly interesting discovery was the labelling of tanycytes, special cells in the brain that monitor metabolism and send signals to eating centres.
Super-resolution microscopy also showed that daLUXendin660 increasingly marks GL1PR and GIPR clusters (‘nanodomains’) in islet cells. Such high-definition images indicate that synergies could arise not only through cumulative signalling pathways, but also through spatially organised receptor clusters.
Limitations of the study include the fact that the markers and the drug tirzepatide are different molecules, and that the investigations have so far been conducted mainly on mouse models. Further research is needed to transfer the results to humans. In addition, only a limited selection of colours was used, but this is to be expanded in the future.
The study therefore provides important insights into why dual agonists are so successful. At the same time, it raises new questions: What would happen if the drugs' access to the brain were improved? And how do new triple agonists with additional glucagon content work?