Scientists of the Max-Born-Institute for Nonlinear in Berlin (Germany) measure directly the spatial positions of electrons and protons during a chemical reaction using ultrashort x-ray flashes.
A chemical reaction generates new compounds out of one or more initial species. On a molecular level, the spatial arrangement of electrons and nuclei changes. While the structure of the initial and the product molecules can be measured routinely, the transient structures and molecular motions during a reaction have remained unknown in most cases. This knowledge, however, is a key element for the exact understanding of the reaction.
The ultimate dream is a "reaction microscope" which allows for an in situ imaging of the molecules during a reaction. The technological challenges of such an ultrafast "cinema" were mastered only recently. Using ultrashort x-ray pulses, researchers at the Max-Born-Institute in Berlin, Germany, now made a "molecular movie" of a chemical reaction with a resolution on atomic length (10‑10meters) and time scales (10‑13 seconds).
Michael Woerner, Flavio Zamponi, Zunaira Ansari, Jens Dreyer, Benjamin Freyer, Mirabelle Premont-Schwarz und Thomas Elsaesser report on a direct time-resolved observation of a chemical reaction in ammonium sulfate crystals [(NH4)2SO4] in the most recent issue of The Journal of Chemical Physics [1]. Using an advanced femtosecond laser system, they generate a blue pulse of 50 femtosecond duration (1 fs = 10‑15 seconds) which initiated the chemical reaction. After a very short period they probe the structure of the excited material with high spatial resolution using a synchronized x-ray flash with only 100 fs duration [2]. The x-ray pulse is diffracted off a powder made of small crystallites (the so called Debye-Scherrer method [3]). Measuring simultaneously many different x-ray reflections the physicists could reconstruct the transient distances of atomic lattice planes and in turn the three-dimensional distribution of electronic charge within the crystal. Taking x-ray snap shots at various delay times after triggering the reaction, they created a molecular movie according to the well known stroboscope effect.
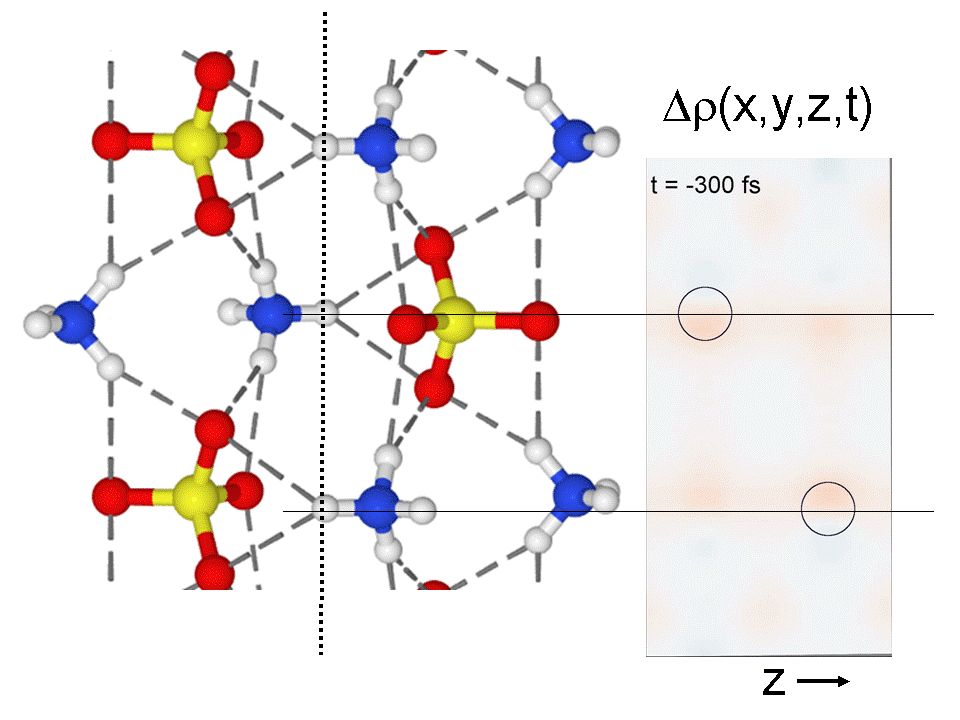
Surprisingly, they observed a reversible chemical reaction which is fundamentally different from the already known, slow thermal phase transitions of ammonium sulfate. First, the blue flash caused a release of both a proton (positive charge) from the ammonium ion (NH4)+ and an electron (negative charge) from the sulfate ion (SO4)-. The two elementary particles merged to a hydrogen atom which jumps back and forth between two distant spatial positions. The corresponding motion is illustrated in the attached movie which was derived from the x-ray snapshots. The circles indicate the initial positions of the protons. The red blots show the motion of the hydrogen atoms after the chemical reaction.
Femtosecond x-ray powder diffraction demonstrated here for the first time can be applied to many other systems, for instance for investigating molecular magnets or for monitoring electron motions in (bio)molecular light harvesting complexes used in solar cells.
Image Caption:
Crystal structure of ammonium sulfate (yellow: sulphur, red: oxygen, blue: nitrogen and grey: hydrogen). The protons along the dotted line leave their ammonium ions and merge with an electron stemming from sulfur atoms to a new hydrogen atom which subsequently jumps back and forth between two spatial positions. This dance takes place in a plane shown on the right hand side. This plane goes through the dotted line and stands perpendicular to the paper in the subfigure on the left hand side.
Here you finde the movie:
http://fvb.preview.internetmanufaktur.de/news/fotos-fuer-news/video-mbi/view
[1] M. Woerner et al., Concerted electron and proton transfer in ionic crystals mapped by femtosecond x-ray powder diffraction, J. Chem. Phys. 133, 064509 (2010).
[2] F. Zamponi, Z. Ansari, M. Woerner, and T. Elsaesser, Femtosecond powder diffraction with a laser-driven hard X-ray source, Opt. Express 18, 947 (2010).
[3] P. Debye and P. Scherrer, Interferenzen an regellos orientierten Teilchen im Röntgenlicht. I., Phys. Zeitschr. 17, 277 (1916).
Contact:
| Dr. Michael Wörner (Tel. 030-6392-1470, email: woerner@mbi-berlin.de), Prof. Thomas Elsaesser (Tel. 030-6392-1400, email: elsasser@mbi-berlin.de) Max-Born-Institut für Nichtlineare Optik und Kurzzeitspektroskopie |