Working with an international team, researchers from the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) have now uncovered a crucial mechanism and elucidated the identity of the axonal transport vesicles that generates synapses. The findings provide an important basis for promoting the regeneration of nerve cells and counteracting the aging process in the future. The results have just been published in the journal Science.
Whether in the brain or in the muscles, wherever there are nerve cells, there are synapses. These contact points between neurons form the basis for the transmission of excitation, i.e. communication between neurons. As in any communication process, there is a sender and a receiver: Nerve cell processes called axons generate and transmit electrical signals thereby acting as signal senders. Synapses are points of contact between axonal nerve terminals (the pre-synapse) and post-synaptic neurons. At these synapses, the electrical impulse is converted into chemical messengers that are received and sensed by the post-synapses of the neighboring neuron. The messengers are released from special membrane sacs called synaptic vesicles.
As well as transmitting information, synapses can also store information. While the structure and function of synapses are comparably well understood, little is known about how they are formed.
A team from the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP)in Berlinhas now shed significant light on this mystery. Scientists from Charité-Universitätsmedizin, the Max Delbrück Center for Molecular Medicine (MDC) and the Universities of Leipzig, Chicago and Sheffield also contributed to this remarkable work.
Fluorescent protein reveals development of synaptic vesicles
To follow the formation of pre-synapses from the beginning, the researchers used CRISPR gene scissors to insert a fluorescent protein into human stem cells, and generated neurons from the modified stem cells. Thanks to the fluorescent marker, the researchers were now able to observe the development of nascent synaptic vesicles in living developing human nerve cells directly under the microscope.
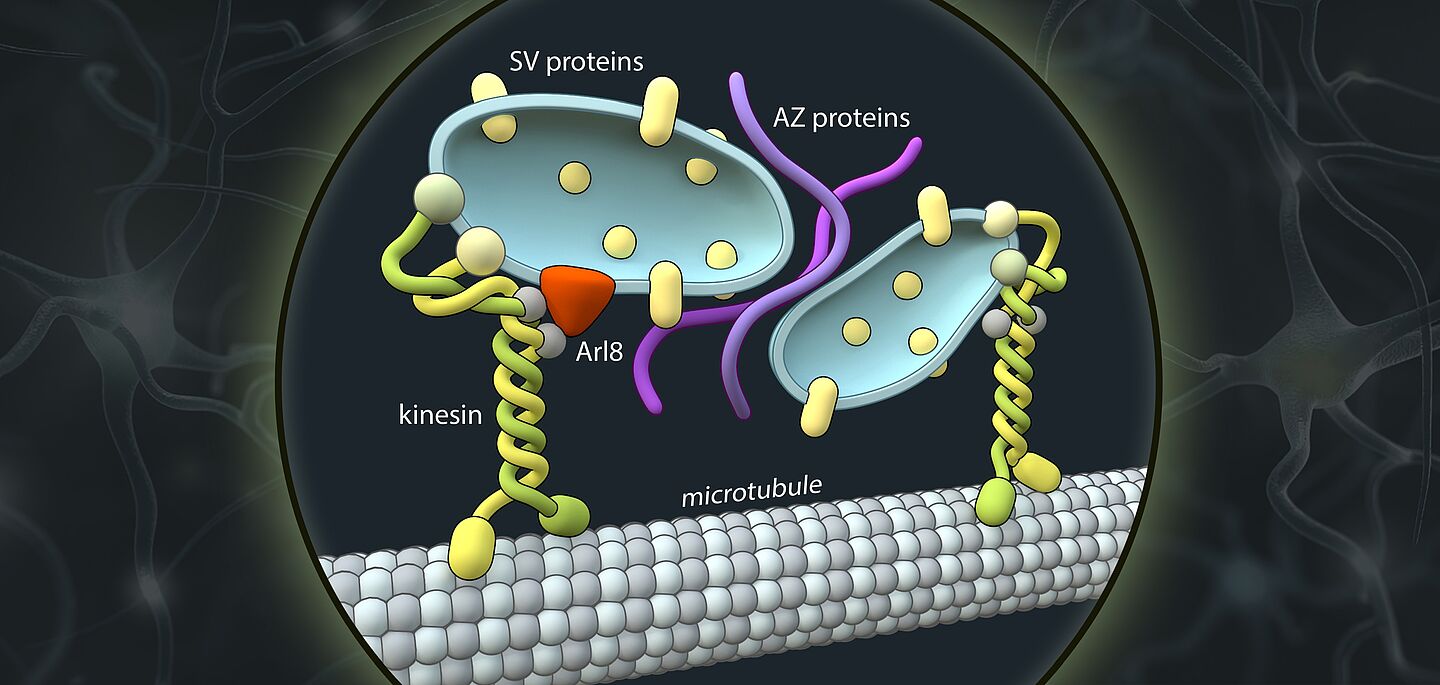
Synaptic vesicles are the membrane vesicles that contain messengers and are stored at each synapse to convert electrical signals into chemical signals. Together with scaffolding proteins that tell synaptic vesicles where the synapse is, and calcium channels that chemically translate the electrical signal, these vesicles form the central elements of the pre-synapse.
All three components have their own genes and are therefore made up of different protein molecules. For this reason, it was previously thought that they also take different routes to ultimately come together in one place to form a functional synapse.
All components set off together
However, the researchers’ observations speak against this hypothesis. “The synaptic vesicle proteins and the proteins of the so-called ‘active zone’ and likely also the adhesion proteins that hold synapses together, share the same bus,” states research group leader Professor Dr. Volker Haucke, describing the surprising finding. “It was highly controversial. And yet our data in human neurons in culture seem quite clear.”
But how exactly do the proteins get to the site of synapse formation? In their study, the researchers were able to show, for one thing, that a machinery of motor proteins powers axonal transport. According to their findings, the main driver is a kinesin known as “KIF1A”. This motor protein is best known for its association with neurological disorders in the peripheral nervous system and the brain. “We suspect that mutations in KIF1A interfere with axonal transport of pre-synaptic proteins, resulting in neurological symptoms such as movement disorders, ataxia, or mental disability,” Volker Haucke explains.
Moreover, the researchers were also able to determine the cell-biological identity of the axonal carriers. That led to another surprise: While the vast majority of secretory vesicles originate from the so-called Golgi apparatus, the axonal transport vesicles do not contain Golgi markers, but share markers with the endolysosomal system, which typically is involved in the degradation of defective proteins in non-neuronal cells. It was a novel combination of light and high-resolution electron microscopy that allowed the researchers to view the axonal transport vesicles ultrastructurally, enabling them to describe their size and shape.
Discovery of transport organelles that exist only in neurons
“Our work suggests that neurons have invented a new kind of organelle, a transport organelle that may be unique to neurons,” explained Dr. Sila Rizalar, postdoctoral fellow at the FMP and lead author of the study published in Science. “This was as little known as the shared transport pathway.”
The new findings from basic research could one day be useful for clinical applications. After all, when the contact points between neurons break down, whether due to disease, accident, or the aging process, it is important to understand the mechanism of axonal transport and the key proteins involved in order to intervene therapeutically. “Ideally, it will be possible to restore or enhance axonal transport to promote neuronal regeneration or counteract aging,” remarks Volker Haucke.
Although the researchers have now unraveled a key mechanism of synapse formation, many questions remain unanswered, such as how the newly discovered transport organelles are formed, what they are made of, and how they deliver their cargo – synapse molecules – to their destination. It also raises the question of whether, perhaps, lifelong memories are stored using the same axonal transport mechanism that is used to form synapses. These are questions that Volker Haucke and his team are now keen to answer. The prospects are exciting.