The contact areas between nerve cells are called synapses. What happens there lies at the heart of communication between nerve cells. Communication starts with the release of chemical messengers known as neurotransmitters at these synapses. Neurotransmitter-containing synaptic vesicles are involved in this release process, and these vesicles fuse with the cell membrane. This fusion occurs at a specific location within the synapse rather than just anywhere at random. Scientists working at the Leibniz Institute for Molecular Pharmacology (FMP) and their colleagues at the Freie Universität Berlin (FU) succeeded in identifying the molecule, which determines where in the synaptic gap neurotransmitters are released. This solves a great mystery in neuroscience. The results contribute to a better understanding of synaptic transmission and may in future improve our ability to explain pathological processes in the nervous system. Recently, the scientists published their groundbreaking scientific results in the renowned science magazine 'Neuron'.
Whether we speak, run, or think – our nervous system always responds, always works by turning electric signals into chemical information and vice versa. This happens in the areas of contact between nerve cells called synapses. An incoming electrical signal at the synapse triggers the inflow of calcium via voltage-dependent calcium channels. In turn, this calcium inflow leads to the release of chemical messengers (neurotransmitters) within just a few milliseconds. In the blink of an eye, the vesicles fuse with the cell membrane. The adjacent nerve cell will then convert the chemical signal back into an electric signal. Scientists refer to this process as 'synaptic transmission' – an elemental process in living organisms.
It is well-known that many vesicles crowd every synapse. However, the release of neurotransmitters occurs in only a few specific spots. Reminiscent of starting blocks on a cinder track, the collocation of the release sites in relation to the calcium channels seems crucial for the synaptic transmission. In both instances, the proper distance determines how fast the finishing line can be reached. In neurotransmission, it determines how fast the electrical signal can be turned into chemical information. Until recently however, the molecule charting the release sites eluded us.
Space and time are interconnected
Scientists working at the Leibniz Institute of Molecular Pharmacology (FMP) and their colleagues at the Freie Universität Berlin (FU) were able to identify the charting molecule. It is the protein Unc13A, and it is well-known to scientists. The protein was discovered in the 1970s. Whenever this protein malfunctioned in roundworms, the movements of these worms became UNCoordinated, thus earning the protein its name. Given the effect of Unc13 on worms, finding an important function for this protein would have been no surprise to scientists even shortly after its discovery.
FMP neuroscientist Dr. Alexander Walter explains: "We knew that the molecule plays an important role in information transfer because there is no synaptic transmission in its absence. Still, we were not aware that this protein determines the neurotransmitter release site."
Scientists needed close to four years, and they used combinations of various measurements and optical methods to track the protein Unc13A down firmly. Changing the location of Unc13A protein in the synapse, and the distance to the calcium channel with it, also shifted the release site of the neurotransmitter. Moving the protein also changed the temporal course of synaptic transmission equivalent to moving starting blocks relative to the finish line. Depending on the distance, information transfer takes more or less time. This proves that the spatial arrangement of the release sites is tightly coupled to the temporal course of the information flow between nerve cells. Dr. Alexander Walter emphasizes: "Our experiments revealed that the exact positioning ensures that synaptic transmission proceeds at the proper speed. I am sure everybody can imagine how important this is for the accurate communication between nerve cells and for the operating mode of the brain“.
Relevance beyond Basic Research
The discovery contributes significantly to understanding the organization of synaptic transmission. A large gap in the neurosciences has been closed.
The scientists used the fruit fly Drosophila melanogaster for their experiments. However, the protein Unc13 also occurs in higher organisms including humans. Therefore, it is very likely that the principle of defined release sites applies across species.
"We must first understand the basics of synaptic transmission before we will be able to understand pathological changes just as we must understand how a car works before we can repair it", argues neuroscientist Dr. Alexander Walter. For this reason, the identification of the molecule is relevant above and beyond basic research. One day, the discovery may benefit patients with neurological diseases.
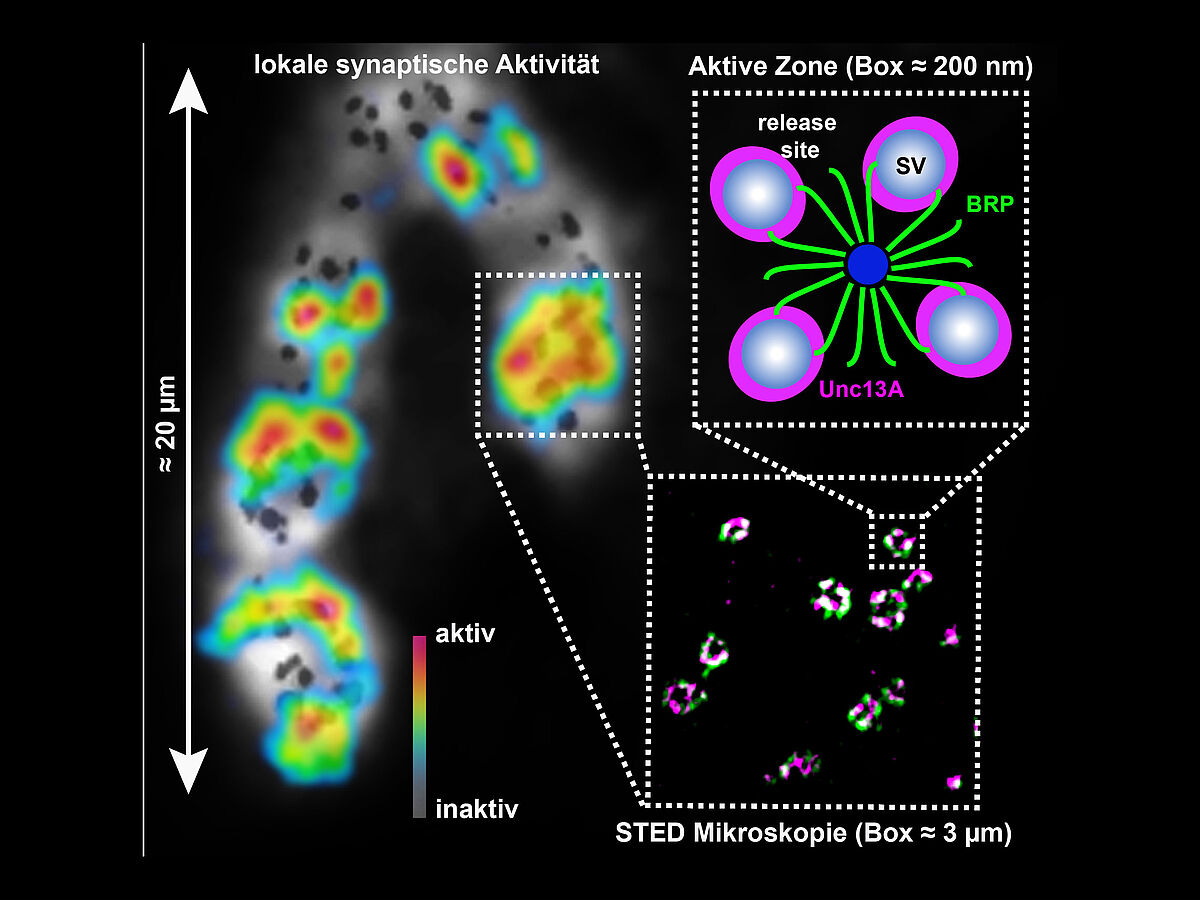
Capture: Neurotransmission: Proteins generate and position 'release sites' for
efficient transmission. Illustration of synaptic activity and structure
on various orders of magnitude starting with the activity pattern (left)
of a neuromuscular end-plate of a fruit fly larva (Drosophila
melanogaster) where neuronal activity is converted into a muscle
contraction. Black dots mark the individual contacts (synapses) between
the neuron and the muscle cell. The activities of the individual
synapses were measured and are depicted in blue (= inactive) to red (=
very active) depending on how active they were. We used a high
resolution (STED) microscope to show the configurations of the so-called
active zones (sites of synaptic activity) in detail. In this case, the
localizing proteins are 'Bruchpilot' (BRP, green) and Unc13A (magenta). A
diagram outlines the protein function in detail: Unc13A generates
release sites where neurotransmitter-containing synaptic vesicles are
released. The protein BRP positions these release sites with high
accuracy for a precisely regulated signal transmission. Illustration:
Mathias Böhme, Andreas Grasskamp, Alexander Walter, FMP.
Source:
Reddy-Alla, S., Böhme, M. A., Reynolds, E., Beis, C., Grasskamp, A. T., Mampell, M.M., Maglione, M., Rey, U., Babikir, H., McCarthy, A. W., Quentin, C., Matkovic, T., Dufour Bergeron, D., Mushtaq, Z., Göttfert, F., Owald, D., Mielke, T., Hell, S. W., Sigrist, S. J., and Walter, A. M. (2017) Stable positioning of Unc13 restricts synaptic vesicle fusion to defined release sites to promote synchronous neurotransmission. Neuron dx.doi.org/10.1016/j.neuron.2017.08.016
Contact:
| Dr. Alexander M. Walter Molecular and Theoretical Neuroscience Leibniz-Institut für Molekulare Pharmakologie (FMP) Charité Campus Mitte Charitéplatz 1, 10117 Berlin Tel.: +49 (0)30-450-639-026 awalterfmp-berlin.de Public Relations Leibniz-Institut für Molekulare Pharmakologie (FMP) Silke Oßwald Tel.: +49 30 94793 104 osswaldfmp-berlin.de |