Theoretical chemistry is a highly interdisciplinary field, encompassing a diverse range of research topics and creating many points of contact between the various natural sciences. Benjamin Philipp Fingerhut from Munich has always placed strong emphasis on interdisciplinarity in his research group at the Max Born Institute (MBI). As a theoretical chemist, he explores the quantum dynamics of molecular systems in order to understand biochemical processes. His theoretical investigations of biomolecular dynamics are an excellent complement to the experimental research being pursued by other workgroups at the Institute.
Funded by the Emmy Noether Program and an ERC Starting Grant
An European Research Council (ERC) Starting Grant received in 2018 provided him with excellent conditions for his research. The prestigious European financial instrument gives young researchers the opportunity to conduct research projects and to establish an independent research program at an early stage of their scientific career. After completing his PhD at Ludwig-Maximilians-Universität München (LMU) and subsequently working there and at the University of California as a postdoc, Benjamin Fingerhut arrived at MBI Berlin in 2014. Funding from the German Research Foundation’s Emmy Noether Program enabled him to establish his research group there.
Using quantum physics to investigate biochemical processes
Using the resources provided by the ERC Starting Grant, he set priorities in two areas in particular. One is developing algorithms to simulate the dynamics of chemical processes according to the fundamental laws of quantum physics. The other is researching physico-chemical interactions at biological interfaces. As fortune would have it, rapid advances in laser technology have made it possible to study even ultrafast biochemical processes experimentally in the laboratory. As a long-standing world leader in this field, MBI is an ideal place for combining theoretical and experimental studies.
When it comes to developing algorithms, Benjamin Fingerhut is interested in simulating systems that are as realistic as possible. Under laboratory conditions, molecules are constantly in contact with their environment and interacting with other molecules and solvents present. Since the laws of quantum physics were originally formulated for isolated systems without external perturbations, they are best suited for calculating the quantum dynamics of undisturbed systems.
However, it is the very interactions with the environment that determine the real events that happen in complex chemical processes. Especially when large and complex biomolecules are involved, interactions of varying strengths occur on multiple levels: sometimes quantum effects are visible; at other times they are obscured by interfering factors like thermal fluctuations.
In order to duly represent these different levels, there are simulation methods in theoretical chemistry that factor in both the quantum mechanical properties and the interactions with the environment. These come into play, for example, when studying energy conversion as occurs in plant photosynthesis. Yet, simulations like these are computationally very intensive and consume a large amount of computing power. Therefore, the research group is working on parallelizing these simulation methods as much as possible, so that they can run on many computer processors simultaneously.
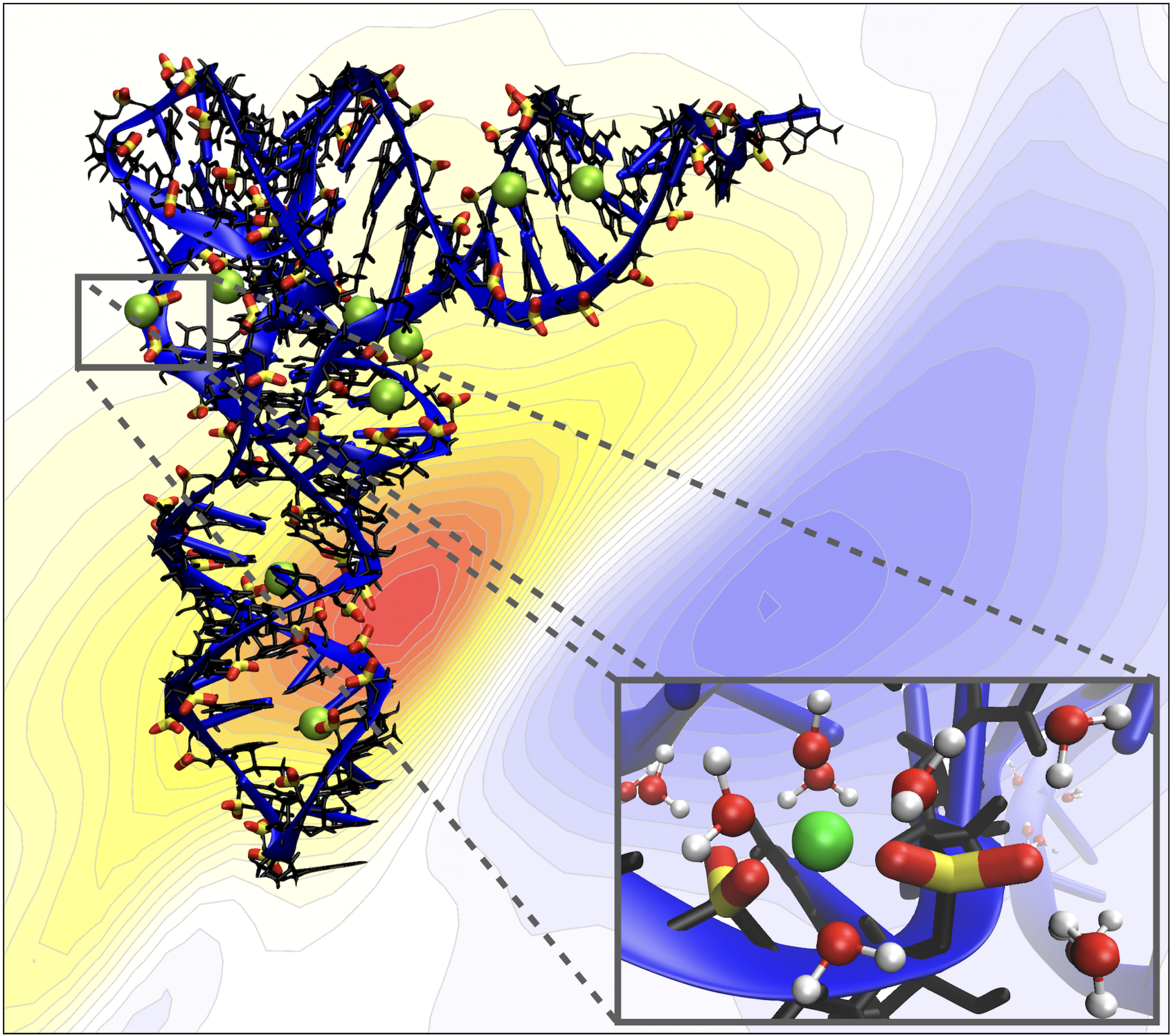
The research group’s second area of interest is the interaction, specifically, of biological interfaces with their aqueous environment, which is another cutting-edge field of research. Although there is an increasing amount of data on the structure of proteins and functional RNA molecules, their three-dimensional structure is most often determined by X-ray crystallography, which looks at samples in crystallized form, which is not how they occur in nature. Therefore, it is not always clear how these biomolecules perform their functions in the natural, aqueous working environment of cells.
Ion interactions with biomolecules
As a good example, positive ions dissolved in water will bind to specific sites on negatively charged RNA molecules. A particularly important functional RNA molecule is transfer RNA, a key molecule in protein synthesis. It delivers the appropriate amino acids for the production of enzymes. It has long been known that if magnesium ions are missing, transfer RNA loses its effectiveness, meaning it can no longer fulfil its function. As the theoretical results of Benjamin Fingerhut’s group show, magnesium ions in water have the special ability to bind directly to the electrically charged phosphate backbone of transfer RNA and thereby stabilize it. It also turns out that this requires only a handful of magnesium ions per molecule of transfer RNA.
These results were confirmed by the experimental research group of MBI’s director, Professor Thomas Elsaesser. Apparently, transfer RNA has evolved in such a way that it always depends on a small number of magnesium ions to strengthen its backbone. In the future, Benjamin Fingerhut would like to extend these analyses to the binding sites of various RNA molecules with proteins.
He is now continuing his career elsewhere, and has in fact come full circle. As of June 2022, he is Professor of Theoretical Chemistry at his alma mater, LMU. However, he remains true to his field of work in continuing to develop algorithms for chemical dynamics and the dynamics of biological interfaces.
Dirk Eidemüller